Newland’s Law of Octaves
In the year 1864, the British chemist John Newlands attempted the 62 elements known at that time. He arranged them in an ascending order based on their atomic masses and observed that every 8th element had similar properties. On the basis of this observation, Newland’s law of octaves was formulated.
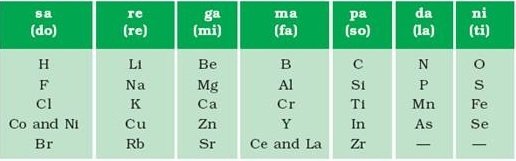
The law of octaves states that every eighth element has similar properties when the elements are arranged in the increasing order of their atomic masses. An illustration detailing the elements holding similar properties as per Newland’s law of octaves is provided below.
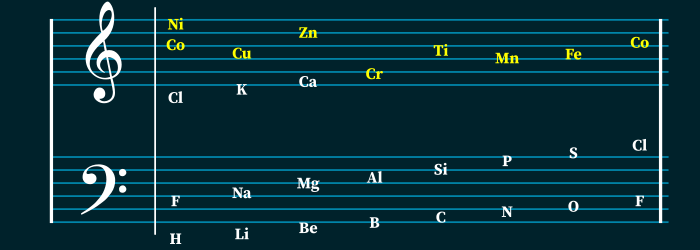
Newlands compared the similarity between the elements to the octaves of music, where every eighth note is comparable to the first. This was the first attempt at assigning an atomic number to each element. However, this method of classifying elements was met with a lot of resistance in the scientific community.
Limitations of Newland’s Law of Octaves
The key shortcomings of Newland’s law of octaves are listed below.
- Several elements were fit into the same slots in Newland’s periodic classification. For example, cobalt and nickel were placed in the same slot.
- Elements with dissimilar properties were grouped together. For example, the halogens were grouped with some metals such as cobalt, nickel and platinum.
- Newland’s law of octaves held true only for elements up to calcium. Elements with greater atomic masses could not be accommodated into octaves.
- The elements that were discovered later could not be fit into the octave pattern. Therefore, this method of classifying elements did not leave any room for the discovery of new elements.
