Modern Periodic Table of Elements
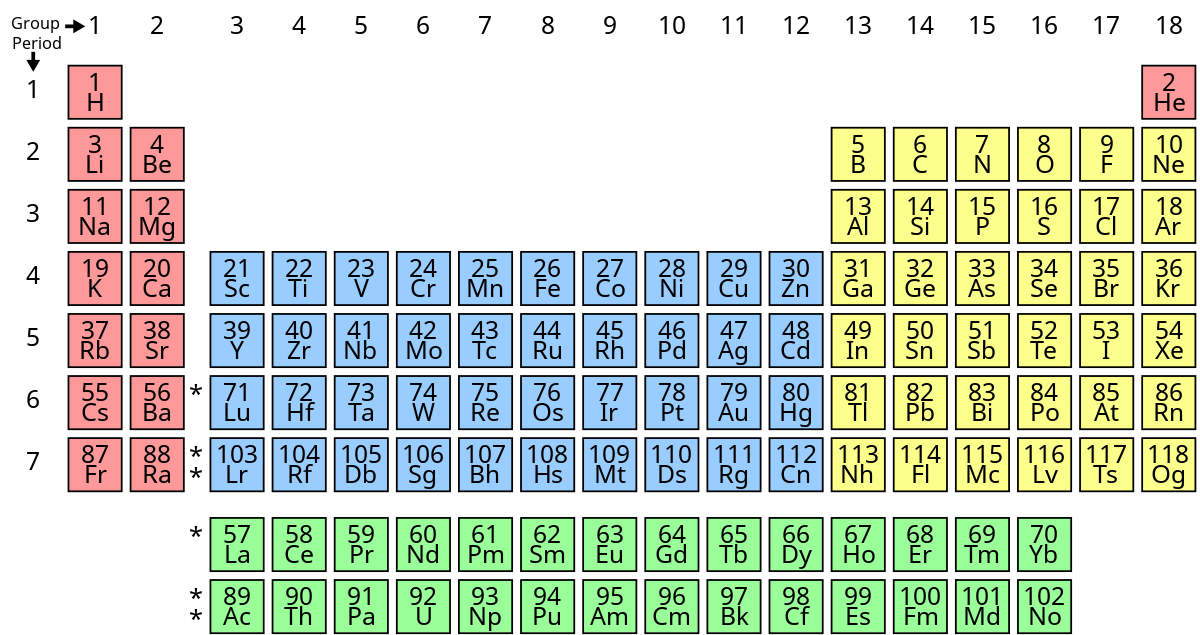
Modern Periodic Law
According to this law:
Chemical properties of elements are periodic function of their atomic number.
This law clearly says that all the chemical properties depend on the atomic number. This is because atomic number decides the electronic configuration, which decides the chemical combination behavior of an element. Of course there are other factors also, but valency depends largely on electronic configuration only.
Limitations of Modern Periodic Table
Following are the limitations of modern periodic table:
1. The Position of Hydrogen is not satisfactory as its properties are similar to both Group 1 and Group 17
2. No separate position given for Isotopes
3. It fails to accommodate Inner transition elements (Lanthanides and Actinides) in its main body.
Trends in Periodic Table
Atomic Size: Atomic size decreases from left to right in a period due to increase in the positive charge in nucleus of atom.
Atomic size increases from top to bottom in a group due to addition of a new shell at each step.
Metallic and non-metallic character: Metallic character decreases and non-metallic character increases from left to right in a period due to increase in hold of nuclues on its outer electron due to increase in positive charge.
Metallic character increases and non-metallic character decreases from top to bottom in a group due to decrease in hold of nuclues on its outer electron due to addition of a shell at each step.
Valency: Valency first increases and then decreases in a period from left to right.
Valency remains same from top to bottom in a group.
