Mendeleev's Periodic Table
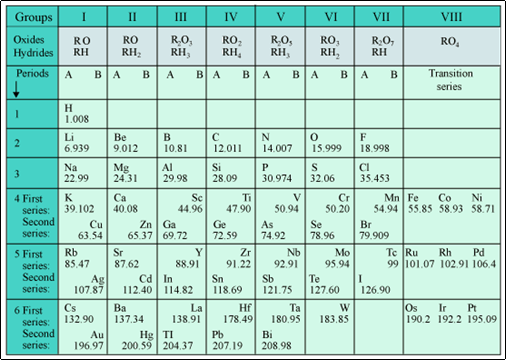
Mendeleev's Periodic Law
According to Mendeleev's periodic law:
All the chemical properties of elements are periodic function of their atomic masses.
Achievements of Mendeleev's Periodic table
(a) The achievements of Mendeleev's periodic table were:
- Mendeleev kept some blank spaces in the periodic table for the elements that were yet to be discovered.
Predicted element
Actual element discovered later
Eka-boron Scandium
Eka-aluminium Gallium
Eka-silicon Germanium
- He also predicted properties of some elements even before their discovery and were later found to be correct.
Property Eka-aluminium Gallium
Atomic mass 68 69.7
Formula of oxide E2O3 Ga2O3
Formula of chloride ECl3 GaCl3
- Mendeleev’s periodic table could accommodate noble gases when they were discovered.
(b) Mendeleev took the formulae of the oxides and hydrides formed by the elements as the basic properties of elements for their classification in the periodic table.
Limitations of Mendeleev's Periodic Table
- He was unable to locate hydrogen in the periodic table.
- Increase in atomic mass was not regular while moving from one element to another. Hence, the number of elements yet to be discovered was not predictable.
- Later on, isotopes of elements were found which violated Mendeleev’s periodic law.
